Coronavirus (COVID-19) Resource Center
Get the latest information on COVID-19 and receiving care at Allina Health.
*Allina Health requires an in-person or virtual visit to confirm if you need a COVID-19 or other tests based on your symptoms. Allina Health does not perform rapid testing. Schedule an appointment to meet with an Allina Health provider to discuss your symptoms and see if you qualify for a COVID-19 test.
Learn more about COVID-19 testing and screening at Allina Health.
Masks are strongly encouraged
Wearing a mask in our clinics and hospitals is strongly recommended for patients and visitors when there is an increase in respiratory illness statewide (Minnesota, Wisconsin).
Wearing a mask is for your protection and the protection of others. If you are coming in for care and also have a recent COVID-19 exposure, cough, runny nose, sore throat or fever, please wear a mask.
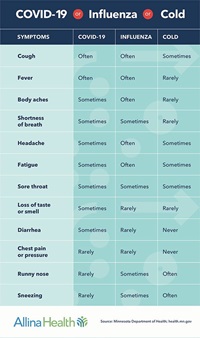
Is it COVID-19, Influenza or a Cold?
Respiratory illnesses like COVID-19, influenza (flu) and the common cold, are spread by:
- Droplets released into the air by coughing or sneezing
- Having unclean hands and touching your face, mouth, eyes or nose
Go to the COVID-19 vs Flu vs Cold chart to see the difference.
Need more guidance or want to find out if COVID-19 testing is recommended for you? Complete a virtual or in-person visit with a care provider.
Getting care for COVID-19
There are multiple ways to get care for common COVID-19 symptoms at Allina Health. If you are experiencing severe symptoms, call 911.